Effects of Upadacitinib on Patient-Reported Outcomes: Results from SELECT-BEYOND, a Phase 3 Randomized Trial in Patients with Rheumatoid Arthritis and Inadequate Responses to Biologic Disease-Modifying Antirheumatic Drugs
Arthritis Res Ther . 2019 Dec 2;21(1):263. doi: 10.1186/s13075-019-2059-8.
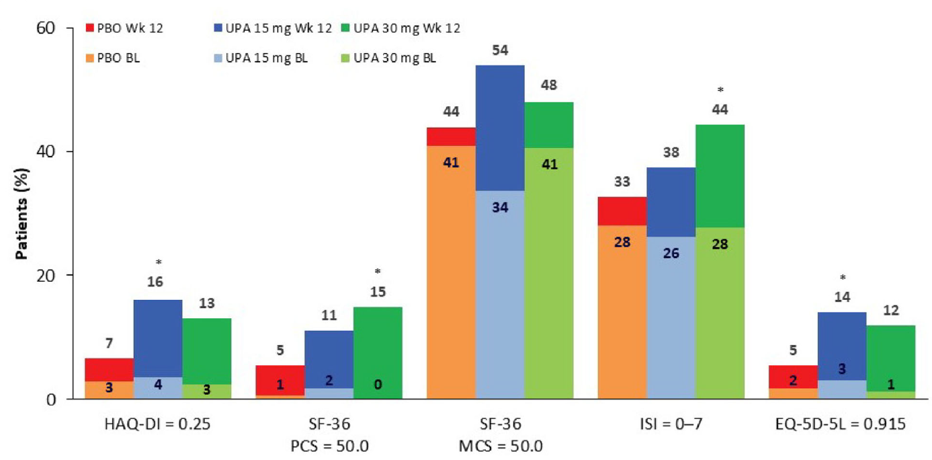
In SELECT-BEYOND, UPA demonstrated clinically meaningful improvements in patient reported outcomes compared to PBO in patients with RA who had an inadequate response to bDMARDs. In this post-hoc analysis of the SELECT-BEYOND study, the effect of UPA 15 or 30 mg on patient reported outcomes were assessed and compared to PBO.Eligible patients (498) with an inadequate response to bDMARDs were randomly assigned 1:1:1 to receive UPA 15 mg, UPA 30 mg or PBO. PROs collected at Wk1, Wk4, and Wk12 included patient global assessment of disease activity, HAQ DI, pain, sleep, morning stiffness, Euro Qol 5-Dimension 5-Level Questionnaire and SF 36. The percentage of patients with improvements in PROs greater than the minimum clinically important difference or scores greater than normative values were compared between UPA and PBO groups.UPA 15 and 30 mg resulted in significant improvements compared to PBO in; patient global assessment of disease activity pain, HAQ-DI, sleep, and the Euro Qol 5-Dimension 5-Level Questionnaire, and all SF-36 domains except mental health and role-emotional.The authors concluded UPA 15 mg or UPA 30 mg improved multiple aspects of QoL with more patients reaching clinically meaningful improvements approaching normative values compared with PBO.