Changes in serum creatinine in patients with active rheumatoid arthritis treated with tofacitinib: results from clinical trials
Arthritis Res Ther. 2014;16(4):R158
Serum creatinine values and renal adverse event data were pooled from patients who received =1 dose of tofacitinib either with background DMARDs or as monotherapy in five Phase 3 studies and two on-going long-term extension studies. Data from five Phase 2 studies were also assessed.
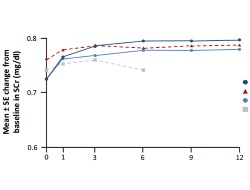
Mean increases in serum creatinine levels of 0.07 mg/dl and 0.08 mg/dl for 5 mg and 10 mg BID tofacitinib doses, respectively, were seen predominantly within the first 3 months of treatment. In the tofacitinib 5 mg BID group, 1.4% had a confirmed increase of ≥33% from baseline in Months 0–3, as did 1.9% of patients in the tofacitinib 10 mg BID group. The changes occurred regardless of whether treatment was as monotherapy or with background DMARDs. No further increases in serum creatinine were seen in these patients during the long-term extension studies.
Clinical acute renal failure (ARF), reported as an adverse event, was seen in 22 tofacitinib-treated patients across the studies, mainly linked to concurrent illnesses associated with ARF in the general population, and was reversible on discontinuation of treatment.
Analyses of pooled data from Phase 2 studies suggested a dose–response effect of tofacitinib on mean serum creatinine in patients with RA. Furthermore, exploratory analysis highlighted a link between these increases and CRP level; the higher the CRP, the greater the increases seen in serum creatinine upon treatment with tofacitinib.
The mechanism behind these changes in serum creatinine in RA patients treated with tofacitinib remains unresolved.