Incidencia de Eventos Tromboembólicos Arteriales y Venosos Reportados en Los Programas de Desarrollo de Tofacitinib en Artritis Reumatoide, Psoriasis y Artritis Psoriásica y de Datos de Mundo Real
Mease P,
Charles-Schoeman C,
Cohen S,
Fallon L,
Woolcott J,
Yun H,
Kremer J,
Greenberg J,
Malley W,
Onofrei A,
Kanik KS,
Graham D,
Wang C,
Connell C,
Valdez H,
Hauben M,
Hung E,
Madsen A,
Jones TV,
Curtis JR
Annals of the Rheumatic Diseases 05 August 2020 2020 Nov;79(11):1400-1413. doi: 10.1136/annrheumdis-2019-216761. Epub 2020 Aug 5.
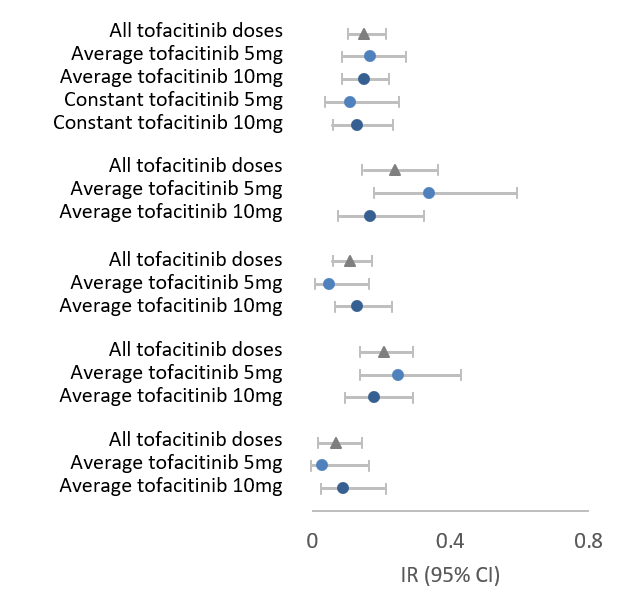
Concerns surrounding increased rates of PE and cardiovascular associated deaths has led to black box warnings when prescribing JAK inhibitors. As such, ongoing investigations regarding cardiovascular and VTE event risks in JAK inhibitor therapies, both clinical and real-world, are vital. Mease and colleagues consider data from clinical tofacitinib development programmes, and the ongoing real-data study A3921133. Conclusions from data analysis state that those with pre-existing cardiovascular and VTE risks show greater IRs of associated events with tofacitinib use.This post-hoc analysis considered both clinical and observational safety data looking at RA and PsA. Overall considerations were made for individual disease-states as opposed to pooling data together.RA data looked at phase 1 to 3, 3b/4 and LTE tofacitinib clinical studies, considering BID and once daily treatment either as monotherapy or in combination with csDMARDs. Comparisons of tofacitinib against placebo, adalimumab and MTX demonstrated similar IRs for a range of cardiovascular and VTE risks. Furthermore, IRs investigated were similar in both tofacitinib 5mg and 10mg BID groups. PsA data considered phase 3 and LTE tofacitinib clinical studies, looking at immediate release tofacitinib in combination with one csDMARD. Results were overall the same as in the previously discussed RA group. Furthermore, both RA and PsA groups demonstrated IRs of multiple cardiovascular and VTE risks to be generally higher in patients with a baseline risk factor versus patients without. However, it should also be considered that the observational study A3921133 demonstrated a higher IR for PE with 10mg tofacitinib in patients with a baseline cardiovascular risk factor.